Figure_2
Renee Matthews
2025-02-21
Last updated: 2025-08-18
Checks: 7 0
Knit directory: ATAC_learning/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20231016) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 9604fa3. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .RData
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/H3K27ac_integration_noM.Rmd
Ignored: data/ACresp_SNP_table.csv
Ignored: data/ARR_SNP_table.csv
Ignored: data/All_merged_peaks.tsv
Ignored: data/CAD_gwas_dataframe.RDS
Ignored: data/CTX_SNP_table.csv
Ignored: data/Collapsed_expressed_NG_peak_table.csv
Ignored: data/DEG_toplist_sep_n45.RDS
Ignored: data/FRiP_first_run.txt
Ignored: data/Final_four_data/
Ignored: data/Frip_1_reads.csv
Ignored: data/Frip_2_reads.csv
Ignored: data/Frip_3_reads.csv
Ignored: data/Frip_4_reads.csv
Ignored: data/Frip_5_reads.csv
Ignored: data/Frip_6_reads.csv
Ignored: data/GO_KEGG_analysis/
Ignored: data/HF_SNP_table.csv
Ignored: data/Ind1_75DA24h_dedup_peaks.csv
Ignored: data/Ind1_TSS_peaks.RDS
Ignored: data/Ind1_firstfragment_files.txt
Ignored: data/Ind1_fragment_files.txt
Ignored: data/Ind1_peaks_list.RDS
Ignored: data/Ind1_summary.txt
Ignored: data/Ind2_TSS_peaks.RDS
Ignored: data/Ind2_fragment_files.txt
Ignored: data/Ind2_peaks_list.RDS
Ignored: data/Ind2_summary.txt
Ignored: data/Ind3_TSS_peaks.RDS
Ignored: data/Ind3_fragment_files.txt
Ignored: data/Ind3_peaks_list.RDS
Ignored: data/Ind3_summary.txt
Ignored: data/Ind4_79B24h_dedup_peaks.csv
Ignored: data/Ind4_TSS_peaks.RDS
Ignored: data/Ind4_V24h_fraglength.txt
Ignored: data/Ind4_fragment_files.txt
Ignored: data/Ind4_fragment_filesN.txt
Ignored: data/Ind4_peaks_list.RDS
Ignored: data/Ind4_summary.txt
Ignored: data/Ind5_TSS_peaks.RDS
Ignored: data/Ind5_fragment_files.txt
Ignored: data/Ind5_fragment_filesN.txt
Ignored: data/Ind5_peaks_list.RDS
Ignored: data/Ind5_summary.txt
Ignored: data/Ind6_TSS_peaks.RDS
Ignored: data/Ind6_fragment_files.txt
Ignored: data/Ind6_peaks_list.RDS
Ignored: data/Ind6_summary.txt
Ignored: data/Knowles_4.RDS
Ignored: data/Knowles_5.RDS
Ignored: data/Knowles_6.RDS
Ignored: data/LiSiLTDNRe_TE_df.RDS
Ignored: data/MI_gwas.RDS
Ignored: data/SNP_GWAS_PEAK_MRC_id
Ignored: data/SNP_GWAS_PEAK_MRC_id.csv
Ignored: data/SNP_gene_cat_list.tsv
Ignored: data/SNP_supp_schneider.RDS
Ignored: data/TE_info/
Ignored: data/TFmapnames.RDS
Ignored: data/all_TSSE_scores.RDS
Ignored: data/all_four_filtered_counts.txt
Ignored: data/aln_run1_results.txt
Ignored: data/anno_ind1_DA24h.RDS
Ignored: data/anno_ind4_V24h.RDS
Ignored: data/annotated_gwas_SNPS.csv
Ignored: data/background_n45_he_peaks.RDS
Ignored: data/cardiac_muscle_FRIP.csv
Ignored: data/cardiomyocyte_FRIP.csv
Ignored: data/col_ng_peak.csv
Ignored: data/cormotif_full_4_run.RDS
Ignored: data/cormotif_full_4_run_he.RDS
Ignored: data/cormotif_full_6_run.RDS
Ignored: data/cormotif_full_6_run_he.RDS
Ignored: data/cormotif_probability_45_list.csv
Ignored: data/cormotif_probability_45_list_he.csv
Ignored: data/cormotif_probability_all_6_list.csv
Ignored: data/cormotif_probability_all_6_list_he.csv
Ignored: data/datasave.RDS
Ignored: data/embryo_heart_FRIP.csv
Ignored: data/enhancer_list_ENCFF126UHK.bed
Ignored: data/enhancerdata/
Ignored: data/filt_Peaks_efit2.RDS
Ignored: data/filt_Peaks_efit2_bl.RDS
Ignored: data/filt_Peaks_efit2_n45.RDS
Ignored: data/first_Peaksummarycounts.csv
Ignored: data/first_run_frag_counts.txt
Ignored: data/full_bedfiles/
Ignored: data/gene_ref.csv
Ignored: data/gwas_1_dataframe.RDS
Ignored: data/gwas_2_dataframe.RDS
Ignored: data/gwas_3_dataframe.RDS
Ignored: data/gwas_4_dataframe.RDS
Ignored: data/gwas_5_dataframe.RDS
Ignored: data/high_conf_peak_counts.csv
Ignored: data/high_conf_peak_counts.txt
Ignored: data/high_conf_peaks_bl_counts.txt
Ignored: data/high_conf_peaks_counts.txt
Ignored: data/hits_files/
Ignored: data/hyper_files/
Ignored: data/hypo_files/
Ignored: data/ind1_DA24hpeaks.RDS
Ignored: data/ind1_TSSE.RDS
Ignored: data/ind2_TSSE.RDS
Ignored: data/ind3_TSSE.RDS
Ignored: data/ind4_TSSE.RDS
Ignored: data/ind4_V24hpeaks.RDS
Ignored: data/ind5_TSSE.RDS
Ignored: data/ind6_TSSE.RDS
Ignored: data/initial_complete_stats_run1.txt
Ignored: data/left_ventricle_FRIP.csv
Ignored: data/median_24_lfc.RDS
Ignored: data/median_3_lfc.RDS
Ignored: data/mergedPeads.gff
Ignored: data/mergedPeaks.gff
Ignored: data/motif_list_full
Ignored: data/motif_list_n45
Ignored: data/motif_list_n45.RDS
Ignored: data/multiqc_fastqc_run1.txt
Ignored: data/multiqc_fastqc_run2.txt
Ignored: data/multiqc_genestat_run1.txt
Ignored: data/multiqc_genestat_run2.txt
Ignored: data/my_hc_filt_counts.RDS
Ignored: data/my_hc_filt_counts_n45.RDS
Ignored: data/n45_bedfiles/
Ignored: data/n45_files
Ignored: data/other_papers/
Ignored: data/peakAnnoList_1.RDS
Ignored: data/peakAnnoList_2.RDS
Ignored: data/peakAnnoList_24_full.RDS
Ignored: data/peakAnnoList_24_n45.RDS
Ignored: data/peakAnnoList_3.RDS
Ignored: data/peakAnnoList_3_full.RDS
Ignored: data/peakAnnoList_3_n45.RDS
Ignored: data/peakAnnoList_4.RDS
Ignored: data/peakAnnoList_5.RDS
Ignored: data/peakAnnoList_6.RDS
Ignored: data/peakAnnoList_Eight.RDS
Ignored: data/peakAnnoList_full_motif.RDS
Ignored: data/peakAnnoList_n45_motif.RDS
Ignored: data/siglist_full.RDS
Ignored: data/siglist_n45.RDS
Ignored: data/summarized_peaks_dataframe.txt
Ignored: data/summary_peakIDandReHeat.csv
Ignored: data/test.list.RDS
Ignored: data/testnames.txt
Ignored: data/toplist_6.RDS
Ignored: data/toplist_full.RDS
Ignored: data/toplist_full_DAR_6.RDS
Ignored: data/toplist_n45.RDS
Ignored: data/trimmed_seq_length.csv
Ignored: data/unclassified_full_set_peaks.RDS
Ignored: data/unclassified_n45_set_peaks.RDS
Ignored: data/xstreme/
Untracked files:
Untracked: RNA_seq_integration.Rmd
Untracked: Rplot.pdf
Untracked: Sig_meta
Untracked: analysis/.gitignore
Untracked: analysis/Cormotif_analysis_testing diff.Rmd
Untracked: analysis/Diagnosis-tmm.Rmd
Untracked: analysis/Expressed_RNA_associations.Rmd
Untracked: analysis/IF_counts_20x.Rmd
Untracked: analysis/LFC_corr.Rmd
Untracked: analysis/SVA.Rmd
Untracked: analysis/Tan2020.Rmd
Untracked: analysis/making_master_peaks_list.Rmd
Untracked: analysis/my_hc_filt_counts.csv
Untracked: code/Concatenations_for_export.R
Untracked: code/IGV_snapshot_code.R
Untracked: code/LongDARlist.R
Untracked: code/just_for_Fun.R
Untracked: my_plot.pdf
Untracked: my_plot.png
Untracked: output/cormotif_probability_45_list.csv
Untracked: output/cormotif_probability_all_6_list.csv
Untracked: setup.RData
Unstaged changes:
Modified: ATAC_learning.Rproj
Modified: analysis/AC_shared_analysis.Rmd
Modified: analysis/AF_HF_SNPs.Rmd
Modified: analysis/Cardiotox_SNPs.Rmd
Modified: analysis/Cormotif_analysis.Rmd
Modified: analysis/DEG_analysis.Rmd
Modified: analysis/DOX_DAR_heatmap.Rmd
Modified: analysis/H3K27ac_integration.Rmd
Modified: analysis/Jaspar_motif.Rmd
Modified: analysis/Jaspar_motif_ff.Rmd
Modified: analysis/SNP_TAD_peaks.Rmd
Modified: analysis/Supp_Fig_12-19.Rmd
Modified: analysis/TE_analysis_ALL_DAR.Rmd
Modified: analysis/TE_analysis_norm.Rmd
Modified: analysis/final_four_analysis.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/Figure_2.Rmd) and HTML
(docs/Figure_2.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 9604fa3 | reneeisnowhere | 2025-08-18 | wflow_publish("analysis/Fig*.Rmd") |
| html | f51c8f5 | reneeisnowhere | 2025-08-18 | Build site. |
| html | 722979b | reneeisnowhere | 2025-08-07 | Build site. |
| Rmd | d5b0a5a | reneeisnowhere | 2025-08-07 | wflow_publish("analysis/Figure_2.Rmd") |
| html | a197856 | reneeisnowhere | 2025-05-01 | Build site. |
| html | b735dc1 | E. Renee Matthews | 2025-02-24 | Build site. |
| Rmd | d88843c | E. Renee Matthews | 2025-02-24 | wflow_publish("analysis/Figure_2.Rmd") |
| Rmd | 8161256 | E. Renee Matthews | 2025-02-21 | next figure |
package loading
library(tidyverse)
library(kableExtra)
library(broom)
library(RColorBrewer)
library("TxDb.Hsapiens.UCSC.hg38.knownGene")
library("org.Hs.eg.db")
library(edgeR)
library(limma)
library(rtracklayer)
library(ggfortify)
library(readr)
library(BiocGenerics)
library(gridExtra)
library(scales)
library(BiocParallel)
library(ggpubr)
library(genomation)
library(ggsignif)
library(plyranges)
library(ggrepel)
library(ComplexHeatmap)
library(cowplot)
library(smplot2)
library(readxl)
library(circlize)
library(epitools)Figure 2
knitr::include_graphics("assets/Figure_2.png", error=FALSE)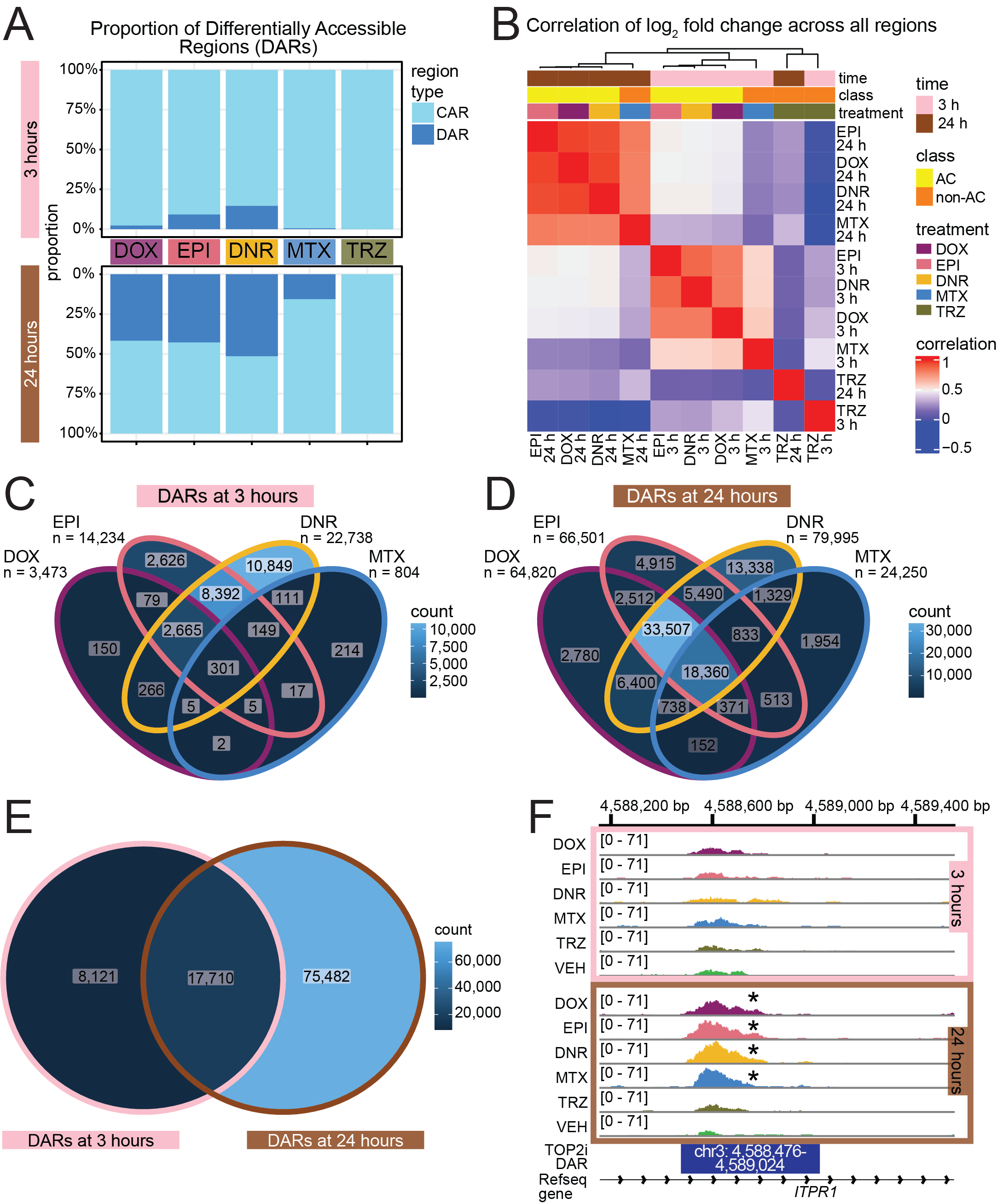
knitr::include_graphics("docs/assets/Figure_2.png",error = FALSE)
Figure 2.A. Proportion of Differentially Accessible Regions (DARs)
toptable_results <- readRDS("data/Final_four_data/re_analysis/Toptable_results.RDS")
all_results <- toptable_results %>%
imap(~ .x %>% tibble::rownames_to_column(var = "rowname") %>%
mutate(source = .y)) %>%
bind_rows()
all_results_list <- toptable_results %>%
imap(~ .x %>% tibble::rownames_to_column(var = "rowname") %>%
mutate(source = .y))
sig_meta_and_loc <- all_results %>%
dplyr::filter(adj.P.Val<0.05) %>% ## filter by pvalue
##Create parsed dataframe from "rowname" column, "genes column will keep id"
separate(rowname, into = c("seqnames", "start", "end"), sep = "\\.", convert = TRUE)
###split into lists by DNR_3, etc..
sig_meta_and_loc_split <- split(sig_meta_and_loc, sig_meta_and_loc$source)
notsig_meta_and_loc <- all_results %>%
dplyr::filter(adj.P.Val>0.05) %>%
separate(rowname, into = c("seqnames","start","end"), sep = "\\.", convert=TRUE)
notsig_meta_and_loc_split <- split(notsig_meta_and_loc, notsig_meta_and_loc$source)
all_DAR_regions <- all_results %>%
separate(rowname, into = c("seqnames", "start", "end"), sep = "\\.", convert = TRUE)
all_DAR_regions_list <- split(all_DAR_regions, all_DAR_regions$source)
three_hour_df <- all_results %>%
dplyr::select(source, genes, logFC,adj.P.Val) %>%
mutate(sig_val=if_else(adj.P.Val<0.05,"sig","not_sig")) %>%
separate(source, into=c("trt","time"),sep="_") %>%
dplyr::filter(time=="3") %>%
mutate(trt=factor(trt, levels=c("DOX","EPI","DNR","MTX","TRZ")))
twentyfour_hour_df <- all_results %>%
dplyr::select(source, genes, logFC,adj.P.Val) %>%
mutate(sig_val=if_else(adj.P.Val<0.05,"sig","not_sig")) %>%
separate(source, into=c("trt","time"),sep="_") %>%
dplyr::filter(time=="24") %>%
mutate(trt=factor(trt, levels=c("DOX","EPI","DNR","MTX","TRZ")))making the figure:
# Ensure consistent trt ordering across both dataframes
ordered_trt <- c("DOX", "EPI", "DNR", "MTX", "TRZ") # adjust to match your treatment order
# 3-hour plot
plot_3h <- three_hour_df %>%
mutate(
sig_val = factor(sig_val, levels = c("not_sig", "sig")),
trt = factor(trt, levels = ordered_trt)
) %>%
ggplot(aes(x = trt, fill = sig_val)) +
geom_bar(position = "fill") +
theme_bw() +
ggtitle("3 hours") +
ylab("Proportion") +
scale_y_continuous(labels = scales::percent)
# 24-hour plot (flipped)
plot_24h <- twentyfour_hour_df %>%
mutate(
sig_val = factor(sig_val, levels = c("not_sig", "sig")),
trt = factor(trt, levels = ordered_trt)
) %>%
ggplot(aes(x = trt, fill = sig_val)) +
geom_bar(position = "fill") +
scale_y_reverse(labels = scales::percent) + # flip it
theme_bw() +
ggtitle("24 hours (flipped)") +
ylab("Proportion")
# Remove x-axis labels from the top plot and y-axis label from the bottom if needed
plot_3h <- plot_3h + theme(axis.title.x = element_blank())
plot_24h <- plot_24h + theme(axis.title.x = element_blank())
# Combine the two using cowplot
combined <- plot_grid(
plot_3h,
plot_24h,
ncol = 1,
align = "v",
axis = "lr", # align left/right axis
rel_heights = c(1, 1)
)
# Show it
print(combined)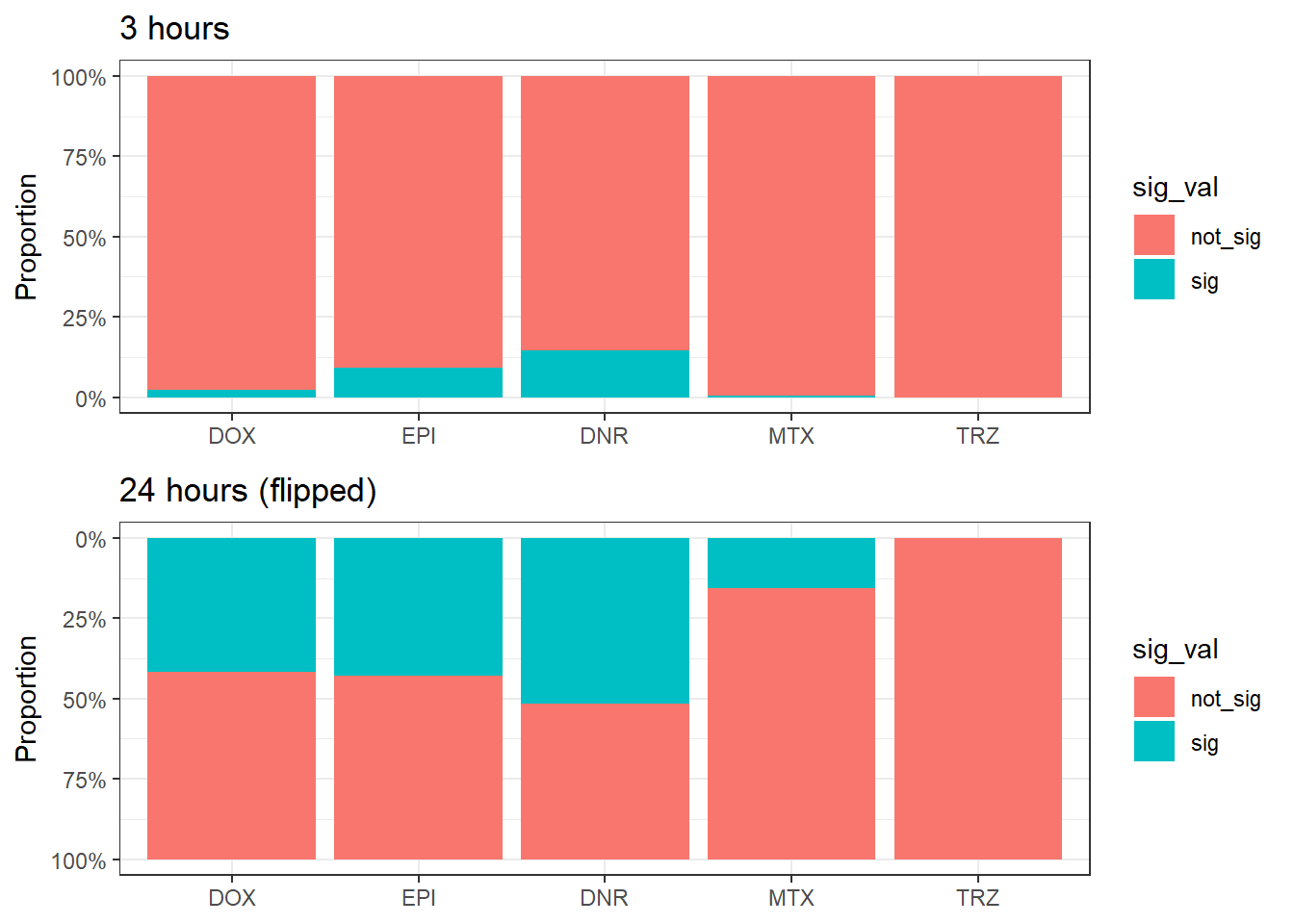
| Version | Author | Date |
|---|---|---|
| 722979b | reneeisnowhere | 2025-08-07 |
Figure 2.B: Log2 Fold change correlation
raw_counts <- read_delim("data/Final_four_data/re_analysis/Raw_unfiltered_counts.tsv",delim="\t") %>%
column_to_rownames("Peakid") %>%
as.matrix()
lcpm <- cpm(raw_counts, log= TRUE)
### for determining the basic cutoffs
filt_raw_counts <- raw_counts[rowMeans(lcpm)> 0,]
filt_raw_counts_noY <- filt_raw_counts[!grepl("chrY",rownames(filt_raw_counts)),]
annotation_mat <- data.frame(timeset=colnames(filt_raw_counts_noY)) %>%
mutate(sample = timeset) %>%
separate(timeset, into = c("indv","trt","time"), sep= "_") %>%
mutate(time = factor(time, levels = c("3h", "24h"))) %>%
mutate(trt = factor(trt, levels = c("DOX","EPI", "DNR", "MTX", "TRZ", "VEH"))) %>%
mutate(indv=factor(indv, levels = c("A","B","C","D"))) %>%
mutate(trt_time=paste0(trt,"_",time))
group <- c( rep(c(1,2,3,4,5,6,7,8,9,10,11,12),4))
group <- factor(group, levels =c("1","2","3","4","5","6","7","8","9","10","11","12"))
dge <- DGEList.data.frame(counts = filt_raw_counts_noY, group = group, genes = row.names(filt_raw_counts_noY))
dge <- calcNormFactors(dge)### this code is not run here, but results from the actual run are saved as an RDS for plotting purposes
group_1 <- c(rep(c("DNR_24","DNR_3","DOX_24","DOX_3","EPI_24","EPI_3","MTX_24","MTX_3","TRZ_24","TRZ_3","VEH_24", "VEH_3"),4))
mm <- model.matrix(~0 +group_1)
colnames(mm) <- c("DNR_24", "DNR_3", "DOX_24","DOX_3","EPI_24", "EPI_3","MTX_24", "MTX_3", "TRZ_24","TRZ_3","VEH_24", "VEH_3")
y <- voom(dge, mm,plot =FALSE)
corfit <- duplicateCorrelation(y, mm, block = annotation_mat$indv)
v <- voom(dge, mm, block = annotation_mat$indv, correlation = corfit$consensus)
fit <- lmFit(v, mm, block = annotation_mat$indv, correlation = corfit$consensus)
cm <- makeContrasts(
DNR_3.VEH_3 = DNR_3-VEH_3,
DOX_3.VEH_3 = DOX_3-VEH_3,
EPI_3.VEH_3 = EPI_3-VEH_3,
MTX_3.VEH_3 = MTX_3-VEH_3,
TRZ_3.VEH_3 = TRZ_3-VEH_3,
DNR_24.VEH_24 =DNR_24-VEH_24,
DOX_24.VEH_24= DOX_24-VEH_24,
EPI_24.VEH_24= EPI_24-VEH_24,
MTX_24.VEH_24= MTX_24-VEH_24,
TRZ_24.VEH_24= TRZ_24-VEH_24,
levels = mm)
fit2<- contrasts.fit(fit, contrasts=cm)
efit2 <- eBayes(fit2)
saveRDS(efit2,"data/Final_four_data/re_analysis/Final_DAR_efit2_w_Bayes.RDS")Plotting the Pearson correlation heat map
efit2 <- readRDS("data/Final_four_data/re_analysis/Final_DAR_efit2_w_Bayes.RDS")
FCmatrix_ff <- subset(efit2$coefficients)
### note coefficients are the LFCs of each region for each contrast. this is just a quick way to pull all that information together to calculate the correlation.
colnames(FCmatrix_ff) <-
c("DNR\n3h",
"DOX\n3h",
"EPI\n3h",
"MTX\n3h",
"TRZ\n3h",
"DNR\n24h",
"DOX\n24h",
"EPI\n24h",
"MTX\n24h",
"TRZ\n24h"
)
mat_col_ff <-
data.frame(
time = c(rep("3 hours", 5), rep("24 hours", 5)),
class = (c(
"AC", "AC", "AC", "nAC","nAC", "AC", "AC", "AC", "nAC","nAC"
)))
rownames(mat_col_ff) <- colnames(FCmatrix_ff)
mat_colors_ff <-
list(
time = c("pink", "chocolate4"),
class = c("yellow1", "lightgreen"))
names(mat_colors_ff$time) <- unique(mat_col_ff$time)
names(mat_colors_ff$class) <- unique(mat_col_ff$class)
# names(mat_colors_FC$TOP2i) <- unique(mat_col_FC$TOP2i)
corrFC_ff <- cor(FCmatrix_ff)
htanno_ff <- HeatmapAnnotation(df = mat_col_ff, col = mat_colors_ff)
Heatmap(corrFC_ff, top_annotation = htanno_ff)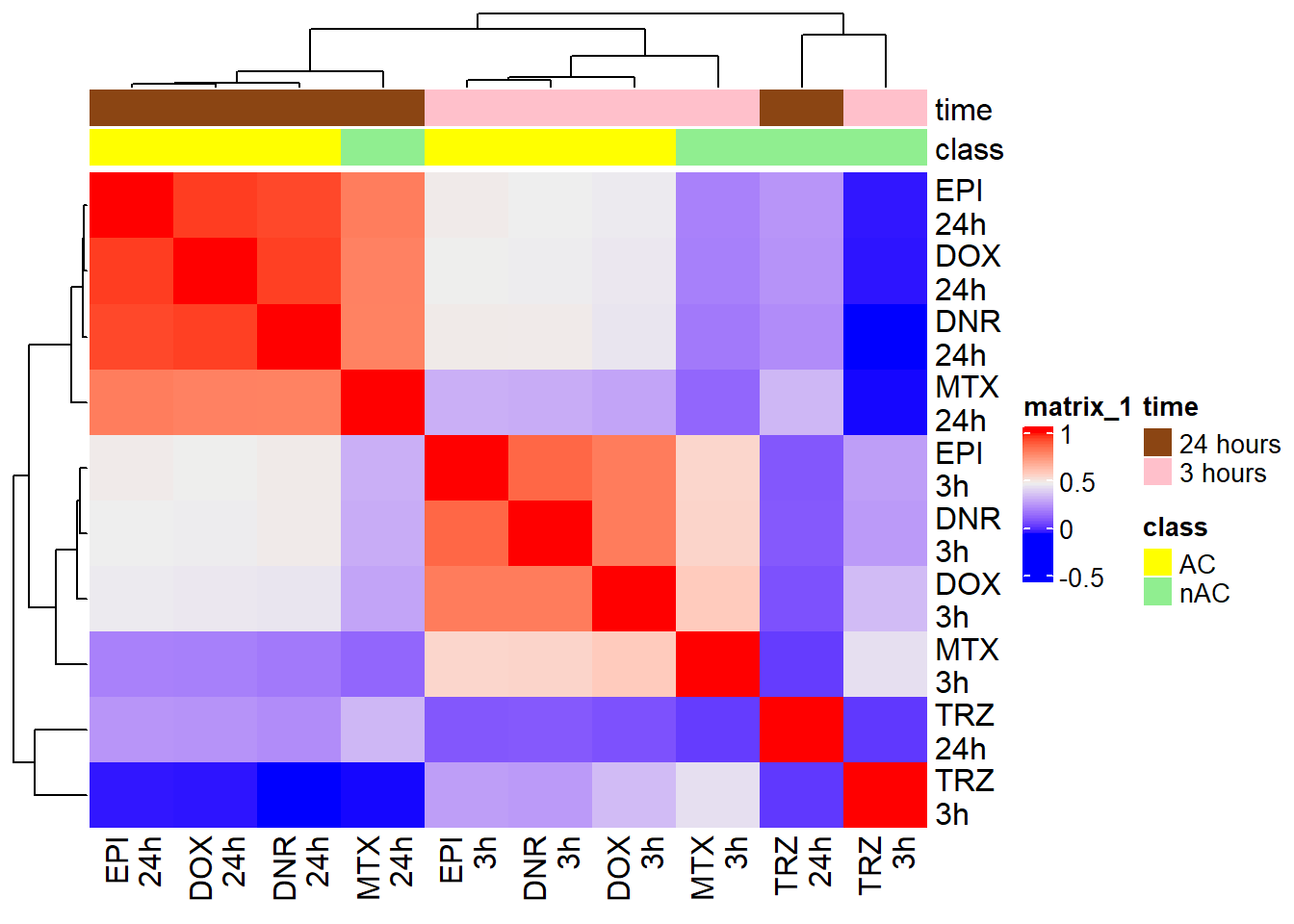
| Version | Author | Date |
|---|---|---|
| 722979b | reneeisnowhere | 2025-08-07 |
Figure 2.C: Venn of DARs at 3 hours
sig_venn_list <- sapply(sig_meta_and_loc_split, function(x) x$genes)
sig_venn_3hr <- sig_venn_list[c("DOX_3","EPI_3", "DNR_3","MTX_3")]
sig_venn_24hr <- sig_venn_list[c("DOX_24","EPI_24", "DNR_24","MTX_24")]
sig_3hr_df <- dplyr::bind_rows(
lapply(names(sig_venn_3hr), function(name) {
data.frame(gene = sig_venn_3hr[[name]], condition = name)
})
) %>%
distinct(gene)
sig_24hr_df <- dplyr::bind_rows(
lapply(names(sig_venn_24hr), function(name) {
data.frame(gene = sig_venn_24hr[[name]], condition = name)
})
) %>%
distinct(gene)
ggVennDiagram::ggVennDiagram(sig_venn_3hr)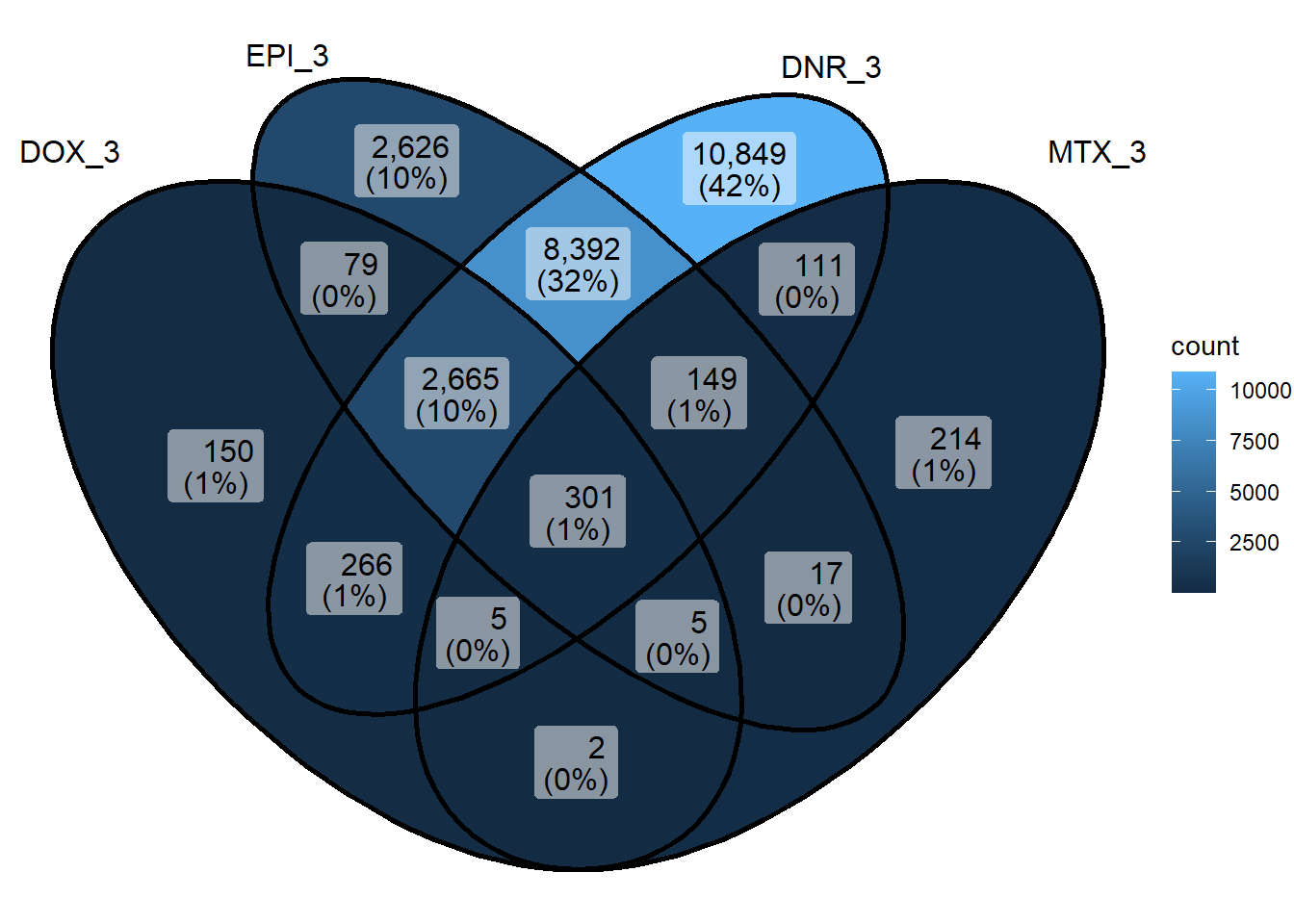
| Version | Author | Date |
|---|---|---|
| 722979b | reneeisnowhere | 2025-08-07 |
Figure 2.D: Venn of DARs at 24 hours
ggVennDiagram::ggVennDiagram(sig_venn_24hr)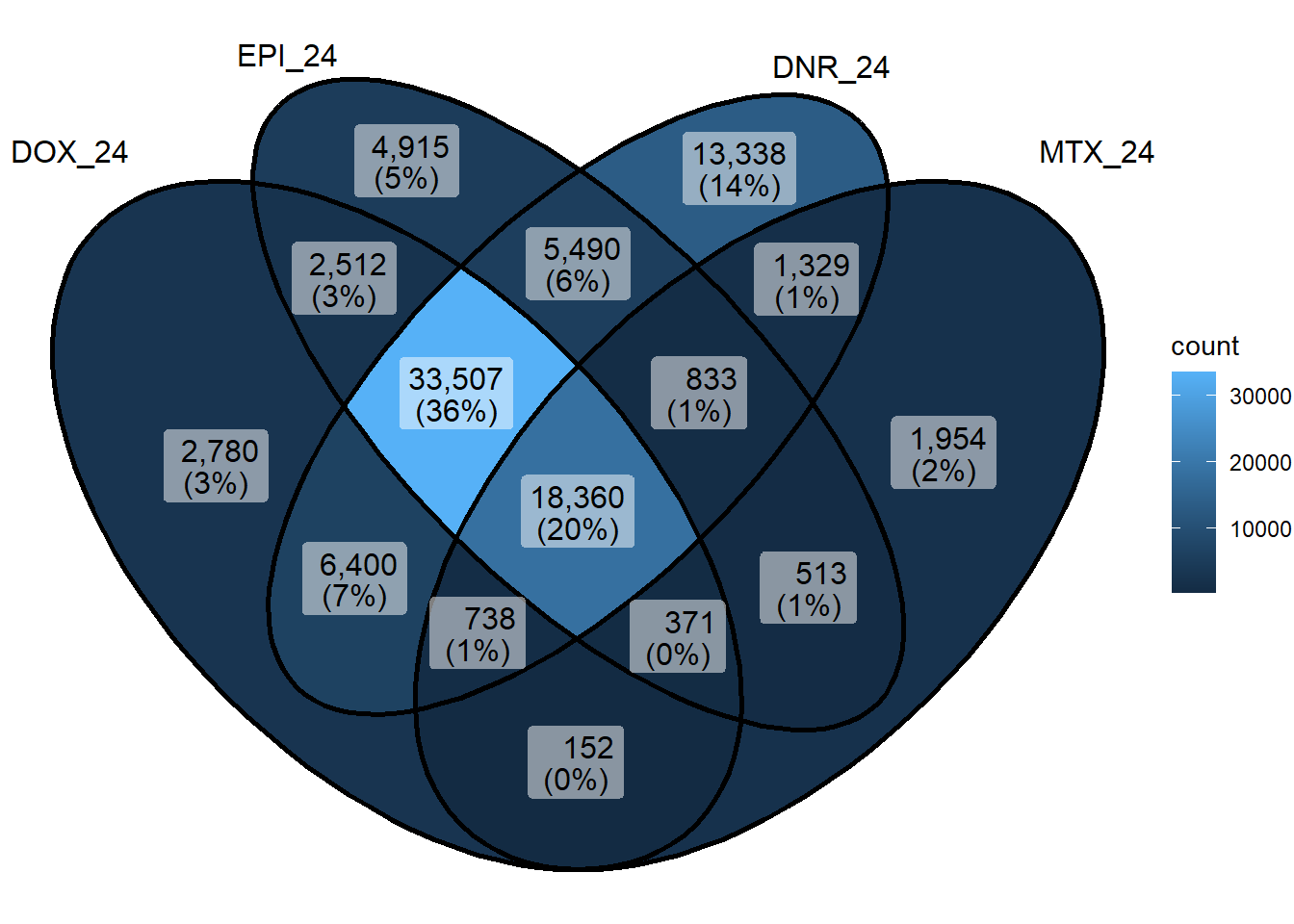
| Version | Author | Date |
|---|---|---|
| 722979b | reneeisnowhere | 2025-08-07 |
Figure 2.E: Venn of DARs between 3 and 24 hours
ggVennDiagram::ggVennDiagram(list("3hr_regions"=sig_3hr_df$gene,"24hr_regions"=sig_24hr_df$gene))+
xlim(-5, 5)+
coord_flip()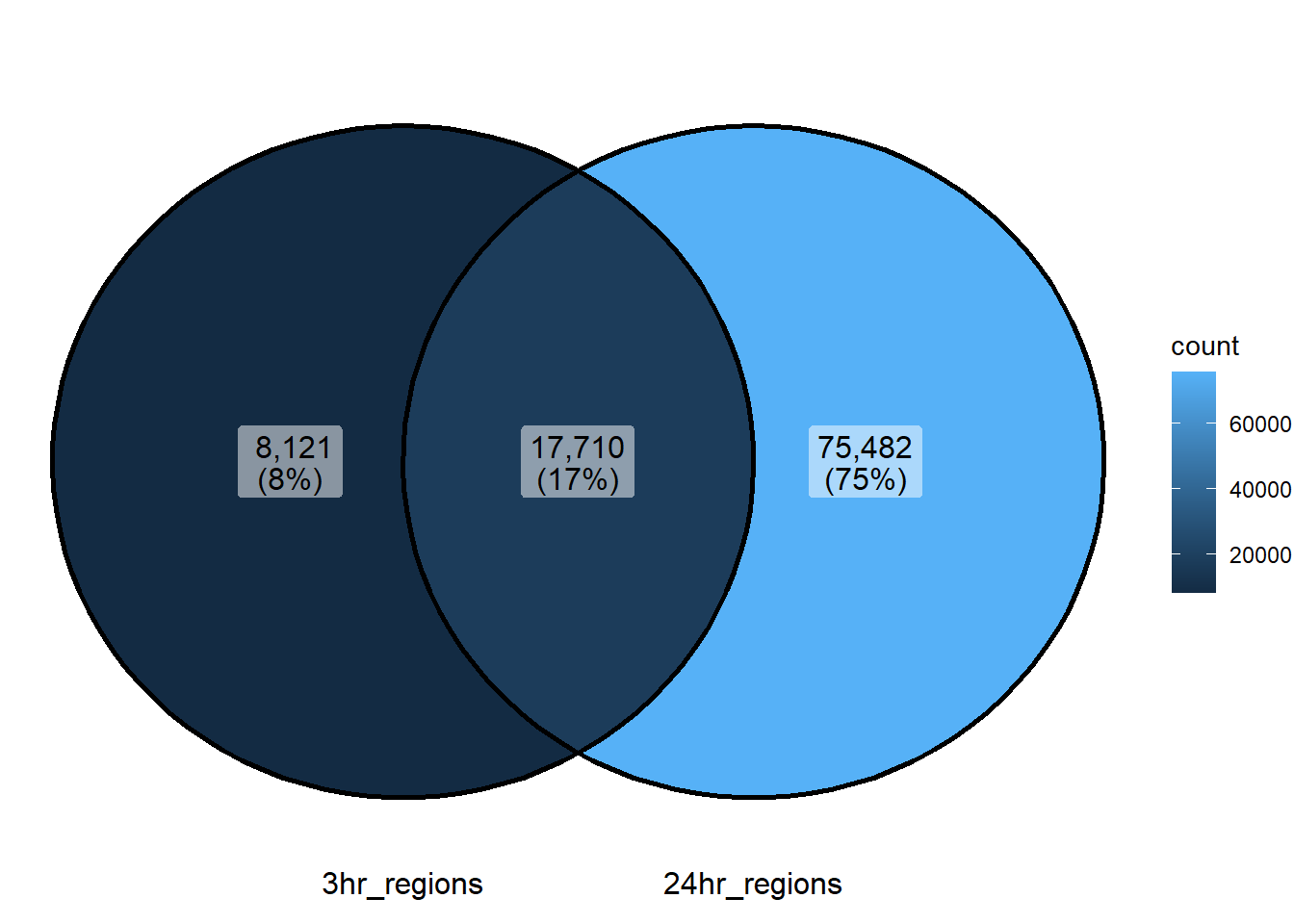
| Version | Author | Date |
|---|---|---|
| 722979b | reneeisnowhere | 2025-08-07 |
Figure 2.F: IGV example of a TOP2if DAR that belongs to 24 hours only DARs
**see image above.
sessionInfo()R version 4.4.2 (2024-10-31 ucrt)
Platform: x86_64-w64-mingw32/x64
Running under: Windows 11 x64 (build 26100)
Matrix products: default
locale:
[1] LC_COLLATE=English_United States.utf8
[2] LC_CTYPE=English_United States.utf8
[3] LC_MONETARY=English_United States.utf8
[4] LC_NUMERIC=C
[5] LC_TIME=English_United States.utf8
time zone: America/Chicago
tzcode source: internal
attached base packages:
[1] grid stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] epitools_0.5-10.1
[2] circlize_0.4.16
[3] readxl_1.4.5
[4] smplot2_0.2.5
[5] cowplot_1.2.0
[6] ComplexHeatmap_2.22.0
[7] ggrepel_0.9.6
[8] plyranges_1.26.0
[9] ggsignif_0.6.4
[10] genomation_1.38.0
[11] ggpubr_0.6.1
[12] BiocParallel_1.40.2
[13] scales_1.4.0
[14] gridExtra_2.3
[15] ggfortify_0.4.19
[16] rtracklayer_1.66.0
[17] edgeR_4.4.2
[18] limma_3.62.2
[19] org.Hs.eg.db_3.20.0
[20] TxDb.Hsapiens.UCSC.hg38.knownGene_3.20.0
[21] GenomicFeatures_1.58.0
[22] AnnotationDbi_1.68.0
[23] Biobase_2.66.0
[24] GenomicRanges_1.58.0
[25] GenomeInfoDb_1.42.3
[26] IRanges_2.40.1
[27] S4Vectors_0.44.0
[28] BiocGenerics_0.52.0
[29] RColorBrewer_1.1-3
[30] broom_1.0.9
[31] kableExtra_1.4.0
[32] lubridate_1.9.4
[33] forcats_1.0.0
[34] stringr_1.5.1
[35] dplyr_1.1.4
[36] purrr_1.1.0
[37] readr_2.1.5
[38] tidyr_1.3.1
[39] tibble_3.3.0
[40] ggplot2_3.5.2
[41] tidyverse_2.0.0
[42] workflowr_1.7.1
loaded via a namespace (and not attached):
[1] later_1.4.2 BiocIO_1.16.0
[3] bitops_1.0-9 cellranger_1.1.0
[5] rpart_4.1.24 XML_3.99-0.18
[7] lifecycle_1.0.4 rstatix_0.7.2
[9] doParallel_1.0.17 rprojroot_2.1.0
[11] vroom_1.6.5 processx_3.8.6
[13] lattice_0.22-7 backports_1.5.0
[15] magrittr_2.0.3 Hmisc_5.2-3
[17] sass_0.4.10 rmarkdown_2.29
[19] jquerylib_0.1.4 yaml_2.3.10
[21] plotrix_3.8-4 httpuv_1.6.16
[23] DBI_1.2.3 abind_1.4-8
[25] zlibbioc_1.52.0 RCurl_1.98-1.17
[27] nnet_7.3-20 git2r_0.36.2
[29] GenomeInfoDbData_1.2.13 svglite_2.2.1
[31] codetools_0.2-20 DelayedArray_0.32.0
[33] xml2_1.3.8 tidyselect_1.2.1
[35] shape_1.4.6.1 UCSC.utils_1.2.0
[37] farver_2.1.2 matrixStats_1.5.0
[39] base64enc_0.1-3 GenomicAlignments_1.42.0
[41] jsonlite_2.0.0 GetoptLong_1.0.5
[43] Formula_1.2-5 iterators_1.0.14
[45] systemfonts_1.2.3 foreach_1.5.2
[47] tools_4.4.2 ggVennDiagram_1.5.4
[49] Rcpp_1.1.0 glue_1.8.0
[51] SparseArray_1.6.2 xfun_0.52
[53] MatrixGenerics_1.18.1 withr_3.0.2
[55] fastmap_1.2.0 callr_3.7.6
[57] digest_0.6.37 timechange_0.3.0
[59] R6_2.6.1 seqPattern_1.38.0
[61] textshaping_1.0.1 colorspace_2.1-1
[63] Cairo_1.6-2 dichromat_2.0-0.1
[65] RSQLite_2.4.2 generics_0.1.4
[67] data.table_1.17.8 htmlwidgets_1.6.4
[69] httr_1.4.7 S4Arrays_1.6.0
[71] whisker_0.4.1 pkgconfig_2.0.3
[73] gtable_0.3.6 blob_1.2.4
[75] impute_1.80.0 XVector_0.46.0
[77] htmltools_0.5.8.1 carData_3.0-5
[79] pwr_1.3-0 clue_0.3-66
[81] png_0.1-8 knitr_1.50
[83] rstudioapi_0.17.1 tzdb_0.5.0
[85] reshape2_1.4.4 rjson_0.2.23
[87] checkmate_2.3.2 curl_6.4.0
[89] zoo_1.8-14 cachem_1.1.0
[91] GlobalOptions_0.1.2 KernSmooth_2.23-26
[93] parallel_4.4.2 foreign_0.8-90
[95] restfulr_0.0.16 pillar_1.11.0
[97] vctrs_0.6.5 promises_1.3.3
[99] car_3.1-3 cluster_2.1.8.1
[101] htmlTable_2.4.3 evaluate_1.0.4
[103] magick_2.8.7 cli_3.6.5
[105] locfit_1.5-9.12 compiler_4.4.2
[107] Rsamtools_2.22.0 rlang_1.1.6
[109] crayon_1.5.3 labeling_0.4.3
[111] ps_1.9.1 getPass_0.2-4
[113] plyr_1.8.9 fs_1.6.6
[115] stringi_1.8.7 viridisLite_0.4.2
[117] gridBase_0.4-7 Biostrings_2.74.1
[119] Matrix_1.7-3 BSgenome_1.74.0
[121] patchwork_1.3.1 hms_1.1.3
[123] bit64_4.6.0-1 KEGGREST_1.46.0
[125] statmod_1.5.0 SummarizedExperiment_1.36.0
[127] memoise_2.0.1 bslib_0.9.0
[129] bit_4.6.0